Amyotrophic lateral sclerosis disease-related mutations disrupt the dimerization of superoxide dismutase 1 - A comparative molecular dynamics simulation study - ScienceDirect

Comprehensive in silico analysis and molecular dynamics of the superoxide dismutase 1 (SOD1) variants related to amyotrophic lateral sclerosis
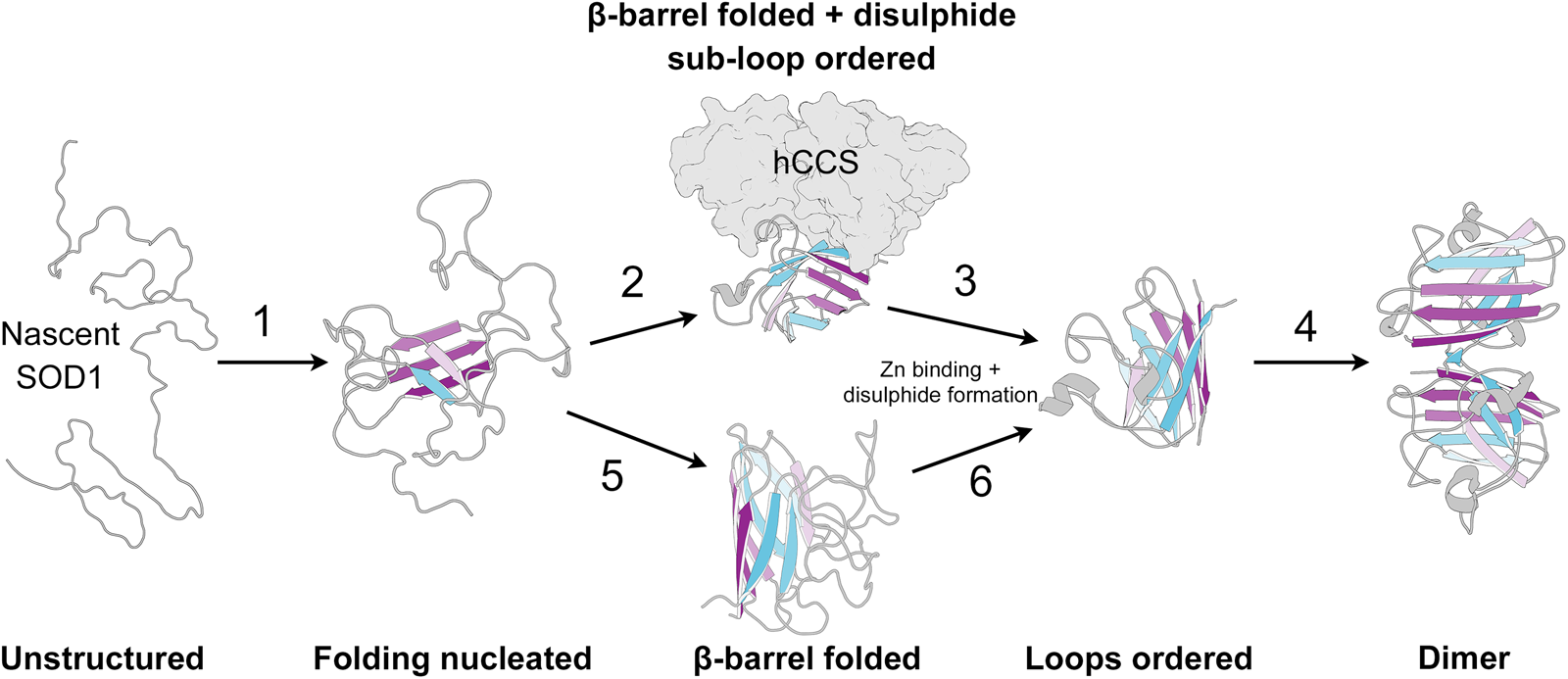
The biophysics of superoxide dismutase-1 and amyotrophic lateral sclerosis, Quarterly Reviews of Biophysics

Full article: Fighting against amyotrophic lateral sclerosis (ALS) with flavonoids: a computational approach to inhibit superoxide dismutase (SOD1) mutant aggregation

Amyotrophic lateral sclerosis disease-related mutations disrupt the dimerization of superoxide dismutase 1 - A comparative molecular dynamics simulation study - ScienceDirect

An Allosteric Pathway in Copper, Zinc Superoxide Dismutase Unravels the Molecular Mechanism of the G93A Amyotrophic Lateral Sclerosis-Linked Mutation

The biophysics of superoxide dismutase-1 and amyotrophic lateral sclerosis, Quarterly Reviews of Biophysics

FRET analysis of WT and SOD1 mutant proteins. A, comparison of WT and
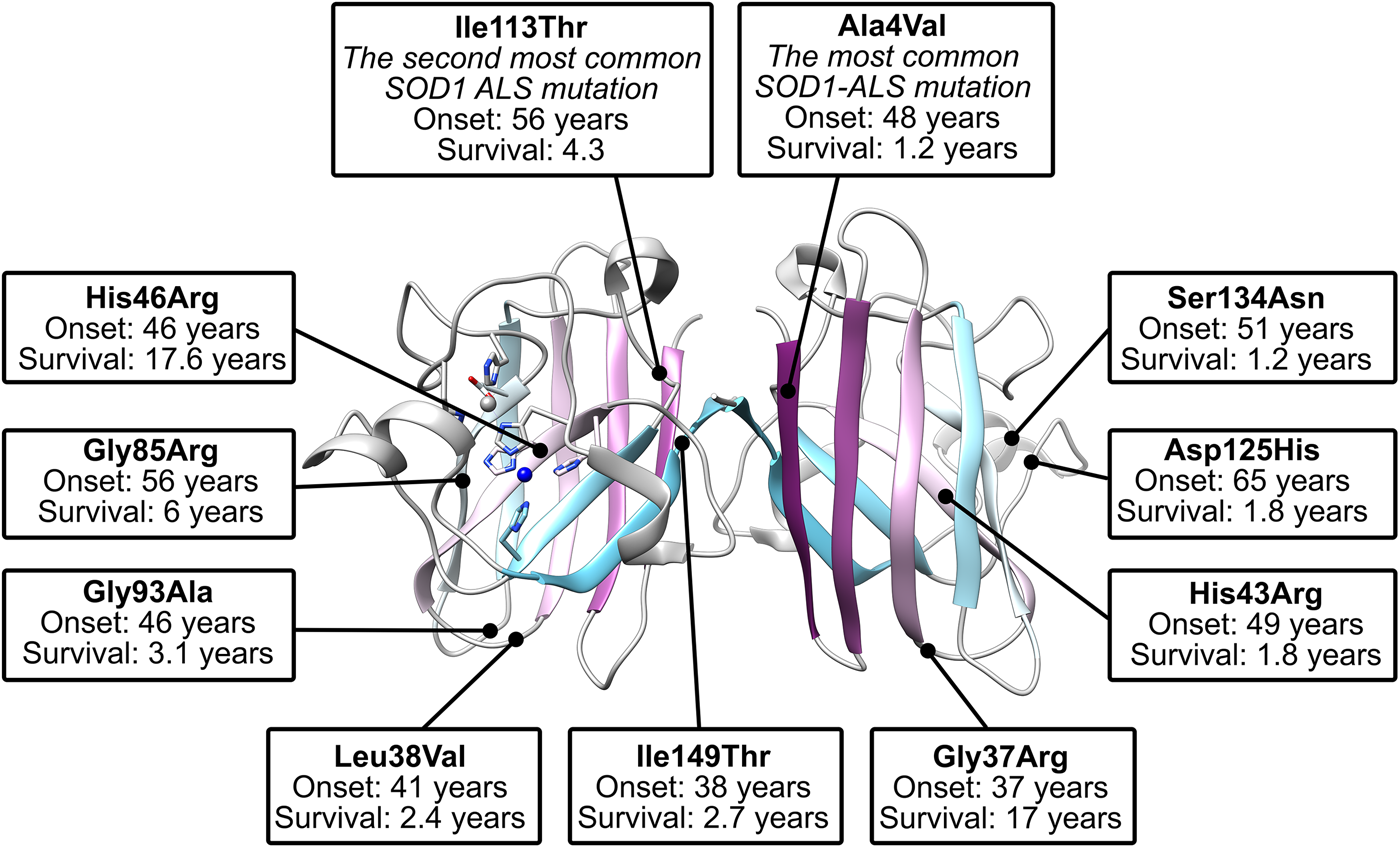
The biophysics of superoxide dismutase-1 and amyotrophic lateral sclerosis, Quarterly Reviews of Biophysics

Amyotrophic lateral sclerosis disease-related mutations disrupt the dimerization of superoxide dismutase 1 - A comparative molecular dynamics simulation study - ScienceDirect